what observations would lead you to believe that the ink is actually a mixture
Chromatography: Be a Color Detective
A colorful project from Science Buddies
Primal concepts
Colors
Solutions
Molecules
Chromatography
Primary colors
Introduction
Practise you lot dearest to use bright and vibrant colored art supplies such as markers or paints? Practise you ever wonder how these colors are made?
The variety of colors comes from colored molecules. These are mixed into the material—whether ink or paint—to brand the product. Some colored molecules are synthetic (or human being-made), such as "Yellow No. v" plant in some nutrient dyes. Others are extracted from natural sources, such as carotenoid (pronounced kuh-RAH-tuh-noid) molecules. These are molecules that make your carrot orange. They can exist extracted from full-bodied natural products, such as saffron.
Only in that location is more to making a colour look the way it does in your homemade artwork. You might take learned that many colors, such every bit orangish and green, are made by blending other, "master" colors. So even though our eyes see a single color, the color of a marker, for instance, might be the result of one type of color molecule or it might be a mix of color molecules responsible. This science activity volition help you discover the hidden colors in water-soluble markers.
Background
We encounter objects considering they reverberate light into our eyes. Some molecules just reflect specific colors; information technology is this reflected, colored light that reaches our eyes and tells our brains that we are seeing a certain color.
Often the colors that nosotros see are a combination of the light reflected by a mixture of unlike-color molecules. Even though our brains perceive the result as ane color, each of the separate types of color molecules stays true to its own color in the mixture. One style to see this is to find a manner to separate out the private types of colour molecules from the mixture—to reveal their unique colors.
Paper chromatography is a method used past chemists to split up the constituents (or parts) of a solution. The components of the solution first out in ane place on a strip of special paper. A solvent (such as water, oil or isopropyl alcohol) is allowed to absorb upwards the paper strip. As it does so, information technology takes function of the mixture with it. Dissimilar molecules run upwardly the newspaper at different rates. Every bit a result, components of the solution separate and, in this case, go visible as strips of color on the chromatography paper. Will your marker ink show different colors as you put it to the test?
Materials
- 2 white coffee filters
- Pair of scissors
- Ruler
- Drawing markers (not permanent): brown, yellow and whatever other colors you would like to test
- At to the lowest degree two pencils (i for each color yous will exist testing)
- At least two alpine water spectacles (i for each color yous will exist testing), four inches or taller
- Water
- Two folder clips or clothespins
- Drying rack or at least two additional tall water spectacles (one for each color you volition be testing)
- Pencil or pen and paper for taking notes
Preparation
- Carefully cut the java filters into strips that are each about one inch wide and at least four inches long. Cut at to the lowest degree two strips, i to test brown and one to test yellow. Cut an extra strip for each additional color you would like to test. How practise y'all expect each of the different colors to behave when you examination it with the paper strip?
- Draw a pencil line beyond the width of each newspaper strip, about one centimeter from the bottom stop.
- Take the chocolate-brown marker and a paper strip and draw a curt line (about one centimeter) on the middle section of the pencil line. Your marker line should not impact the sides of your strip.
- Use a pencil to write the color of the marker you just used on the tiptop stop of the strip. Notation: Do not use the colored marker or pen to write on the strips, equally the color or ink will run during the test.
- Repeat the previous 3 steps with a yellowish marker and then all the boosted colors you lot would like to test.
- Hold a paper strip next to ane of the alpine spectacles (on the outside of it), aligning the elevation of the strip with the rim of the glass, then slowly add together water to the glass until the level just reaches the bottom end of the paper strip. Repeat with the other drinking glass(es), keeping the strips still on the exterior and abroad from the h2o. What part exercise y'all retrieve the water volition play?
Procedure
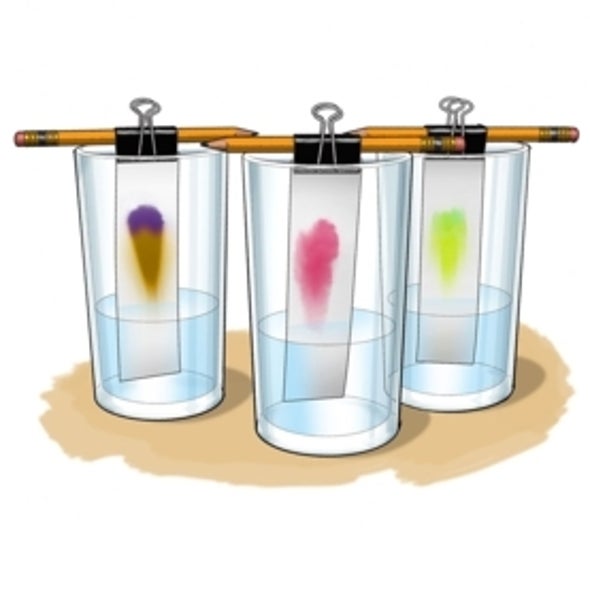
- Spike the peak of a strip (the side farthest from the marker line) to the pencil with a binder clip or clothespin. Break for a moment. Practice y'all look this color to be the result of a mixture of colors or the effect of one color molecule? If you like, you tin can brand a note of your prediction at present.
- Hang the strip in ane of the glasses that is partially filled with h2o by letting the pencil rest on the glass rim. The bottom finish of the strip should just impact the water level. If needed, add water to the glass until it is just touching the paper. Notation: It is of import that the water level stays below the marking line on the strip.
- Go out the offset strip in its drinking glass every bit y'all echo the previous two steps with the second strip and the second glass. Repeat with any additional colors you are testing.
- Lookout as the water rises up the strips. What happens to the colored lines on the strips? Does the color run up likewise? Do you encounter any color separation?
- When the water level reaches nearly one centimeter from the top (this may have up to 10 minutes), remove the pencils with the strips attached from the glasses. If yous let the strips run besides long, the water can reach the elevation of the strips and distort your results.
- Write downwardly your observations. Did the colors run? Did they dissever in different colors? Which colors can you detect? Which colors are on the top (meaning they ran quickly) and which are on the lesser (meaning they ran more than slowly)?
- Hang your strips to dry out in the empty glasses or on a drying rack. Note that some colors might keep running after you remove the strips from the water. Yous might need longer strips to run into the full spectrum of these colors. The notes you lot took in the previous stride volition assistance yous remember what yous could see in case the colors run off the newspaper strip. Await at your strips. How many color components does each marker color accept? Can you identify which colors are the upshot of a mixture of color components and which ones are the outcome of one hue of color molecule? Are individual color components brightly colored or dull in colour? How many different colors can you detect in total?
- Extra: Most watercolor mark inks are colored with synthetic colour molecules. Artists oft like to piece of work with natural dyes. Information technology is adequately easy to make your own dye from colorful plants such equally blueberries, cherry-red beets or turmeric. To make your own dye, have an adult help you finely chop the plant material and identify it in a saucepan. And add simply enough h2o to encompass the plant material. Let the mixture simmer covered on the stove for approximately 10 to 15 minutes. If, at this point, the color of your liquid is likewise faint, you might want to remove the lid of the saucepan and keep boiling until some liquid has evaporated and a more concentrated color is obtained. Let it absurd and strain when needed. Now you take natural dye. (Handle with caution, every bit it can stain surfaces and materials.) To investigate the color components of this dye, repeat the previous process merely supplant the marker line with a driblet of natural dye. A dropper volition help create a overnice drop. Allow the drib of dye dry earlier running the paper strip. Would the colour of your natural dye exist the upshot of a mixture of color molecules or one specific color molecule? Does the marker of the same colour as your natural dye run in a like mode as your natural dye does?
- Extra: In this activity you used water-soluble markers in combination with h2o as a solvent. Y'all tin can test permanent markers using isopropyl rubbing booze as a solvent. Do you retrieve similar combinations of color molecules are used to color similar-colored permanent markers?
- Extra: You tin investigate other art supplies, including paints, pastels or inks in a similar style. Be sure to ever cull a solvent that dissolves the material that is beingness tested to run the chromatography examination. Isopropyl rubbing alcohol, vegetable oil and common salt water are some examples of solvents used to perform paper chromatography tests for different substances.
Observations and results
Did you find that brown is fabricated up of several types of colour molecules, whereas yellow only showed a single yellow colour ring?
Marker companies combine a small-scale subset of colour molecules to make a broad range of colors, much like you tin can mix paints to make dissimilar colors. Only nature provides an even wider range of color molecules and also mixes them in interesting ways. As an example, natural yellow color in turmeric is the result of several curcuminoid molecules. The brown pigment umber (obtained from a dark brown clay) is acquired by the combination of two color molecules: iron oxides (which accept a rusty red-brown color) and manganese oxides (which add a darker black-chocolate-brown color).
In this activity you lot investigated the colour components using coffee filters as chromatography paper. Your colour bands might be quite wide and artistic, whereas scientific chromatography newspaper would yield narrow bands and more-exact results.
Cleanup
Throw away the newspaper strips and wash the spectacles.
More than to explore
Paper Chromatography, from ChemGuide
Paper Chromatography: Is Black Ink Really Black?, from Science Buddies
Make Your Ain Markers, from Science Buddies
Processed Chromatography: What Makes Those Colors?, from Science Buddies
Find the Hidden Colors of Autumn Leaves, from Scientific American
This activity brought to you in partnership with Scientific discipline Buddies
Source: https://www.scientificamerican.com/article/chromatography-be-a-color-detective/
0 Response to "what observations would lead you to believe that the ink is actually a mixture"
Post a Comment